
Research in the Hunter Group
The success of synthetic chemistry in the last century was built on the development of a set of design rules that allowed the quantitative prediction of reactivity and conformation based simply on chemical structure. The goal of our research is to establish a comparable set of rules that can be used for the design of non-covalent systems with equal reliability. This will not only be fundamental science in its own right, relevant to a broad range of chemical problems, but it will also be technology that can be implemented in biological and materials applications.
 Fundamental investigations of the nature of intermolecular interactions: synthetic supramolecular systems are
ideally suited for the systematic study and quantitative determination of the thermodynamic properties of non-covalent
interactions. This research will contribute to our fundamental understanding of non-covalent chemistry by establishing
a sound quantitative basis for assessing the relative contributions of different factors that influence the behaviour
of complex systems. The impact of such intuitive understanding combined with rules and software for predicting the
magnitudes of these effects cannot be underestimated in the context of molecular design.
Fundamental investigations of the nature of intermolecular interactions: synthetic supramolecular systems are
ideally suited for the systematic study and quantitative determination of the thermodynamic properties of non-covalent
interactions. This research will contribute to our fundamental understanding of non-covalent chemistry by establishing
a sound quantitative basis for assessing the relative contributions of different factors that influence the behaviour
of complex systems. The impact of such intuitive understanding combined with rules and software for predicting the
magnitudes of these effects cannot be underestimated in the context of molecular design.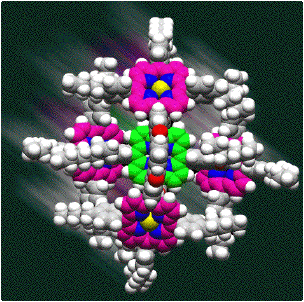 Molecular design of functional assemblies: the ultimate test of a truly integrated understanding of molecular
recognition in complex systems is the ability to undertake a rational design project. Nature has long inspired
chemists with glimpses of the spectacular levels of sophistication and functionality that are possible with
well-organised molecular systems. Among these systems, nucleic acids stand out. In addition to their unique biological
properties, they have found widespread applications in the evolution of receptors and catalysts and as programmable
building materials for nanotechnology. These properties are currently unrivalled in any other material, but we propose
to develop new classes of information molecule that will offer a programmable synthetic alternative that bears no
chemical resemblance to the biological counterpart.
Molecular design of functional assemblies: the ultimate test of a truly integrated understanding of molecular
recognition in complex systems is the ability to undertake a rational design project. Nature has long inspired
chemists with glimpses of the spectacular levels of sophistication and functionality that are possible with
well-organised molecular systems. Among these systems, nucleic acids stand out. In addition to their unique biological
properties, they have found widespread applications in the evolution of receptors and catalysts and as programmable
building materials for nanotechnology. These properties are currently unrivalled in any other material, but we propose
to develop new classes of information molecule that will offer a programmable synthetic alternative that bears no
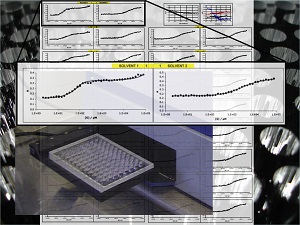
chemical resemblance to the biological counterpart.  Development of new methods for studying solvation and cooperativity and their role in determining
the properties of molecular systems in solution. Solvation and cooperativity are two critical factors that have
complicated the development of a quantitative molecular recognition chemistry. Understanding solvent effects is one of
the great unsolved problems in chemistry, and our combined experimental and theoretical approach is developing
a conceptual framework for a general quantitative understanding of molecular recognition in different solvent
environments. An understanding of how solvents work at a molecular level will be of practical utility to the whole
chemistry community, and it will help to solve one of the major barriers to progress in the development of reliable
computational methods for predicting molecular behaviour. Although cooperativity has been widely studied, it is also
a phenomenon that has proved difficult to predict at a quantitative level. Through systematic structure-activity
studies of cooperative supramolecular systems, we are collecting a large body of experimental data on families of
thousands of closely related complexes. This will provide the fundamental experimental data required to unlock an
understanding of cooperativity at a quantitative level.
Development of new methods for studying solvation and cooperativity and their role in determining
the properties of molecular systems in solution. Solvation and cooperativity are two critical factors that have
complicated the development of a quantitative molecular recognition chemistry. Understanding solvent effects is one of
the great unsolved problems in chemistry, and our combined experimental and theoretical approach is developing
a conceptual framework for a general quantitative understanding of molecular recognition in different solvent
environments. An understanding of how solvents work at a molecular level will be of practical utility to the whole
chemistry community, and it will help to solve one of the major barriers to progress in the development of reliable
computational methods for predicting molecular behaviour. Although cooperativity has been widely studied, it is also
a phenomenon that has proved difficult to predict at a quantitative level. Through systematic structure-activity
studies of cooperative supramolecular systems, we are collecting a large body of experimental data on families of
thousands of closely related complexes. This will provide the fundamental experimental data required to unlock an
understanding of cooperativity at a quantitative level.- Computer modelling of intermolecular interactions: we use energy calculations in conjunction with experimental data such as crystallographic databases to investigate the factors that determine the relationship between chemical structure, three-dimensional structure and the organisation of complex systems. The development of these models will provide tools for understanding the properties and function of a wide range of molecular systems. One important area of application is understanding how DNA sequence is used to program the molecular organisation of the nucleus. As more information on genomic DNA sequences and macromolecular DNA assemblies becomes available, our computational tools will play an increasingly important role in assessing the relationship between DNA sequence, three-dimensional structure and function. There are important applications in understanding the role of epigenetic modifications on the structural properties, and hence function, of DNA.
Techniques involved in our research include organic synthesis, coordination chemistry, NMR spectroscopy, mass spectrometry, X-ray crystallography, high-throughput physical organic chemistry, structural and thermodynamic characterisation of intermolecular complexes, molecular design, molecular modelling, biophysics, bioinformatics and computer programming.

